Theory & Informatics
Reliable and rapid theoretical assessment of reversible caloric systems that involve multiple control fields (e.g., magnetic, stress, electric) is a critical component for accelerating discovery and design of new caloric materials. The objective is to guide experimental synthesis and testing, supplement go/no-go decisions on system selection, develop physics-derived optimization of materials and their response, and provide pertinent data for informatics-based materials design and discovery.
We utilize new and proven theoretical methods and their careful application, particularly reliable electronic-structure codes and thermodynamic models, to guide and support experiment for system down selection, rapid characterization or optimization of materials selection space. For example, calculations offer rapid means (see figure below) to identify chemical modification to optimize properties, evaluate systems for low-energy barriers to control transformations, or simply eliminate systems having low potential for strong caloric response. One focus area is design of fast estimators for thermodynamic information needed to identify promising systems through informatics-based machine-learning methods supported through out partners.
Discovery and Evaluation Activities
- Down select and optimize materials selection space
- Design fast estimators to identify promising systems
- Calculate electronic & thermodynamic properties to support experimental search and informatics
- Evaluate directly the informatics-derived caloric systems
- Assess experimentally-derived systems for characterization and feedback to the informatics
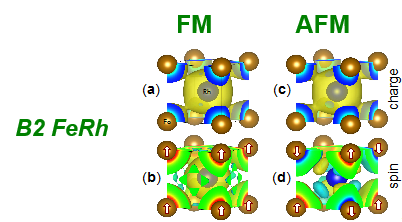
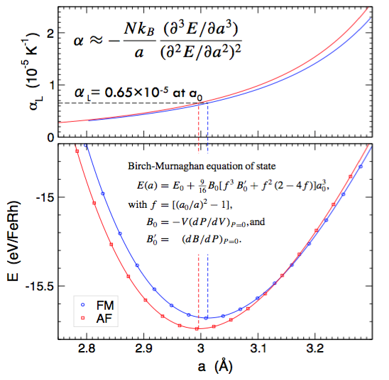
Antiferromagnetic (AFM) and ferromagnetic (FM) FeRh in B2 (or CsCl) structure are well-known and well-studied systems exhibiting strong magneto-caloric and elasto-caloric response. The left-side figure shows the chemical, charge, and spin configuration that compete upon heating to transform from AFM to FM state. The calculated energy in the right-side plot shows the competing AFM and FM states that yields an estimated transition temperature of 346 K (experiment is 353 K). As also shown, the curvature of the energy versus volume provides a fast estimate for the linear thermal expansion (6.5 vs. 6.7±0.3 experimentally in units of 10^-6/K), relevant to the transformation. Other effects, such as phonon entropy, can also be estimated to provide predictions for caloric behavior: theory 11.9 J/kg/K versus 12-14 experimentally.




